| Colin S. Gaines |
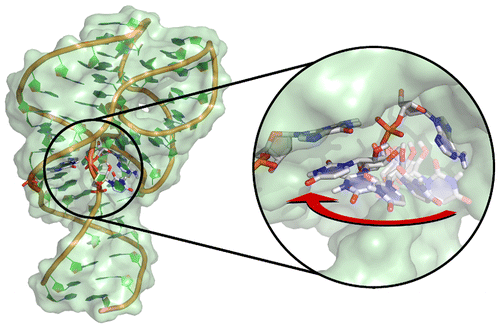
Graduate student Colin Gaines has had his first paper accepted: "Ribozyme Catalysis With A Twist: The Active State Of The Twister Ribozyme In Solution Predicted From Molecular Simulation"
This paper was an effort to fully characterize the catalytically active state of the recently discovered twister ribozyme. In contrast to the available crystal structures, it was shown that a rearrangement of the uracil adjacent to the scissile phosphate (U-1) to stack beneath G33 best represented an active conformation. Molecular dynamics simulations and free energy calculations were performed to explore the likelihood of sampling this conformational state as well as its impact on in-line fitness (a requirement for catalysis). Additionally, our calculations support the hypothesis that two highly conserved residues, A1 and G33, act as the general acid and base. This provided evidence for an intriguing new twist for RNA catalysis - the N3 site of A1 can act as a general acid due to the shifting of its pKa towards neutrality within the active site.
Recently, this paper was featured in C&EN. See it here:
http://cen.acs.org/articles/94/i12/new-twist-revealed-ribozyme-catalysis.html
